06
11/2022
Several evaluations are transcribed, it could be a public policy to access another strategy to contain the spread of the pandemic, improve isolation and avoid or mitigate the wave of propagation. We must be alert to explore alternative paths, how to implement an improvement in equity, in access, and in mitigating pandemic effects.
It is evident that the vaccines in use are essential for containing the pandemic, reducing severe cases and mortality, but their effectiveness decreases very quickly, especially for mutations. I understand that science should develop other more effective vaccine technologies, strategies not only for the prevention of serious and fatal disease, but also for transmission, because otherwise the number of cases, with their respective isolates, will lead to a decrease in the productive force of essential workers, the production of goods, the exchange and replacement of some habits that increase economic growth
by Serge Tonen-Wolyec 1,2,Raphaël Dupont 3,Natalio Awaida 3,Salomon Batina-Agasa 2< /sup>,Marie-Pierre Hayette4 and Laurent Bélec5, *
Due to their ease of use, the lateral flow assay SARS-CoV-2 rapid antigen detection tests could be suitable candidates for the rapid antigen detection self-test (Ag-RDST). We evaluated the feasibility of the BIOSYNEX COVID-19 Ag+ Ag-RDST antigen autotest (Biosynex Swiss SA, Freiburg, Switzerland), using self-collected nasal secretions from the turbinate medium (NMT), in 106 prospectively enrolled adult volunteers. Living in Paris, France. Most of the participants correctly understood the instructions for use (94.4%; 95% confidence interval (CI) 88.3-97.4), showing a great ability to perform the entire self-test procedure to obtain a valid and interpretable result (100%; 95% CI: 96.5-100), and demonstrated the ability to correctly interpret test results (96.2%; 95% CI: 94.2-97.5 ) with a high level of overall satisfaction. Approximately one in eight participants (#15%) required verbal help to perform or interpret the test, and only 3.8% of test results were misinterpreted. Referring to real-time multiplex RT-PCR, Ag-RDST showed 90.9% and 100% sensitivity and specificity, respectively, and high agreement (98.1%), reliability (0.94) and precision. (90.9%) to detect SARS. CoV-2 antigen. Taken together, our study demonstrates the high usability and precision of the BIOSYNEX Antigen Self-Test COVID-19 Ag+ for supervised self-collected NMT sampling in an unselected adult population living in France. and high concordance (98.1%), reliability (0.94), and precision (90.9%) for detecting the SARS-CoV-2 antigen. Taken together, our study demonstrates the high usability and precision of the BIOSYNEX Antigen Self-Test COVID-19 Ag+ for supervised self-collected NMT sampling in an unselected adult population living in France. and high concordance (98.1%), reliability (0.94), and precision (90.9%) for detecting the SARS-CoV-2 antigen. Taken together, our study demonstrates the high usability and precision of the BIOSYNEX Antigen Self-Test COVID-19 Ag+ for supervised self-collected NMT sampling in an unselected adult population living in France.
While currently recommended nucleic acid amplification tests, such as real-time reverse transcription polymerase chain reaction assays, remain the cornerstone of the gold standard for the diagnosis of SARS- CoV-2 [4, 5], immunological methods can also be used to detect viral antigens [5, 6, 7]. Therefore, rapid antigen detection diagnostic tests (Ag-RDTs) enable new testing strategies for the diagnosis and control of SARS-CoV-2 infection due to their short response time and ease of use [ 6, 8, 9]. However, most SARS-CoV-2 Ag-RDTs rely on nasopharyngeal sampling, and their use is hampered by the need to deploy skilled healthcare workers and protective equipment to collect, test, and interpret nasopharyngeal secretions. their results. In addition, a nasopharyngeal swab is often considered uncomfortable, limiting the ability to frequently screen this type of nasopharyngeal sampling [10]. Alternative sampling methods, such as nasal mid-turbinate sampling (NMT) ( a swab is inserted about 2-3 cm into the nostril parallel to the palate until resistance is met in the turbinates), they are well tolerated and can be performed by the individual without supervision at home or supervised in situ [10, 11, 12, 13]. NMT sampling was equivalent to nasopharyngeal sampling for a WHO-listed SARS-CoV-2 Ag-RDT, which lays the foundation for its use as a supervised self-sampling technique [12, 13, 14, fifteen ]. Widespread use of self-tests for SARS-CoV-2 infection could help improve control of the spread of the SARS-CoV-2 epidemic [16, 17, 18, 19, 20, 21]. Previous experience with HIV self-testing showed that a self-test can be performed perfectly by a common person and gives reliable and accurate results, and that HIV self-testing is considered an acceptable and useful tool to improve HIV testing coverage worldwide. the world to improve control of the HIV epidemic [22, 23]. Lateral flow tests for SARS-CoV-2 antigen are appropriate for use as a rapid antigen detection self-test (Ag-RDST) due to their low cost, ease of use, and rapid result [12, 14 , 24]. Recent reports have established the feasibility of self-sampling for NMT under supervision [12, 14]. However, data on the clinical performance of self-testing with Ag-RDT are limited to comparisons with quantitative real-time RT-PCR (rtRT-PCR) detection of SARS-CoV-2 RNA [25, 26]. This study aimed to evaluate the usability and clinical performance in the field of the Ag-RDST BIOSYNEX COVID-19 Ag+ Antigen Self-Test (Biosynex Swiss SA, Freiburg, Switzerland; reference 859271) for COVID-19 antigen testing. of NMT collected by themselves. secretions in adults living in the Paris region during the third wave of the COVID-19 epidemic in France. For the first time to our knowledge, the usability of this novel Ag-RDST was evaluated according to a systematic approach based on our previous experience evaluating HIV and SARS-CoV-2 self-test using capillary blood samples [27, 28, 29, 30].
Table: Blanca Lumbreras (UMH) and Salvador Peiró (Fisabio) Download the data
Study population
A total of 116 people were assessed for eligibility, but 10 were excluded because they were under 18 years of age (n = 4) and did not give consent (n = 6) ( Figure 1 ). Ultimately, 106 were successfully enrolled in all clinical performance evaluation and feasibility substudies. The demographic characteristics and medical history of study participants are shown in Table 1. In total, 68 (64.2%) were women. The median age was 40 years and most of the participants were between 21 and 59 years. The majority (83.9%) of the participants had at least a university or higher education level. The majority (87.7%) of them had no previous experience in self-assessment of diseases other than COVID-19.3.1. The main reasons for testing were air travel (n=41; 38.7%), exposure of a case by contact of an individual infected with SARS-CoV-2 (n=10; 9.4%) , suspected COVID-19 (n = 30; 28.3%) ), preoperative evaluation (n = 18; 17.0%), and control of SARS-CoV-2 infection in the previous 30 days (n = 7 ; 6.6%). At the time of testing, the majority (71.7%) of the participants had not reported symptoms of COVID-19 in the past month, including all contact cases of people infected with SARS-CoV-2. About a third of the participants reported at least one symptom consistent with COVID-19. Among symptomatic patients, the mean duration of symptoms before sampling was 4 days (range, 0 to 7 days).
Discussion
In this paper we report our recent experience during the third peak period of the COVID-19 epidemic in France of the usability and clinical performance in the field of a new rapid antigen test and self-administered Ag-RDSTs for COVID-19 antigenic testing of self-collected NMT secretions from adult volunteers living in the Paris region. Most of the participants were able to correctly understand the instructions for use of the self-test, obtain a reliable result and interpret the final results of the test. Approximately one in eight (#15%) participants required verbal help, and only 3.8% of test results were misinterpreted. Taken together, our study demonstrates the feasibility and accuracy of self-testing for COVID-19 from self-collected NMT samples in an unselected adult population in a supervised setting.
4.1. Feasibility evaluation
The global usability of the BIOSYNEX Antigen Self-Test COVID-19 Ag + was evaluated through four successive substudies that allow a good analysis of all the steps of the test, from the understanding of instructions for use with test identification. components, until the Ag-RDST was performed and the final interpretation of the results. The satisfaction substudy is also essential to assess the acceptability of the self-test, which will necessarily need to be repeated in an epidemic context. Substudy 1 assessed whether all participants could read and understand the instructions for use. Our findings showed that 94.4% of the participants answered all 11 questions correctly, indicating a generally correct understanding of the key messages delivered by the BIOSYNEX Antigen Self-Test COVID-19 Ag+ instructions for use, with a rate overall good responses of 96.8%. These satisfactory results can be partly explained by a sufficient level of education of most of the study participants. In fact, previous experience with HIV self-testing has shown that an insufficient level of education is a great challenge in understanding the instructions for use [22, 40]. Although systematic reviews and meta-analyses have shown that HIV self-testing can be successfully performed by untrained users without assistance [22], our observations underscore the need to supplement traditional paper-based instructions with other educational tools, such as a short video , which was preferred by 28.3% of the study participants for a better understanding of the instructions for use. These findings are reminiscent of previous WHO recommendations for HIV self-assessment, that all self-assessors should have the option of accessing assistance by phone, the Internet, or additional instructions such as videos, animations, or diagrams [41]. The difficulty of explaining that the swab must touch the lower part of the inner wall of the nostril for correct NMT sampling should be emphasized. This difficulty, found in 5.6% of the participants, could be the cause of false-negative results. In substudy 2, all study participants performed the COVID-19 Ag-RDST and managed to obtain a test result. valid with a general usability index estimated at 99.1%. The test time was particularly short (8.1 min). Some difficulty in correctly inserting the nasal swab into both nostrils and the need to rotate the swab 6 times in the diluent tube were the main reported concerns encountered and were the most common reason for oral aid. All participants who used the video instructions performed the self-test easily. These features underscore the importance of video instructions, when available. The use of a hotline could also provide direct remote assistance. The ability to correctly read and interpret self-assessment results is considered a delicate step in self-assessment [42]. This refers not only to visual ability related to good visual acuity when reading and interpreting the results, but also to the intensity of the bands to be read on the strip. In our series, the rate (96.2%) of correct interpretation of the COVID-19 Ag-RDST results was high, as is often seen, for example, with HIV self-testing with a similar cassette [28, 29, 30 ]. In one case, misinterpreted test results referred to a faint positive band, read as negative. This difficulty in reading some faint positive bands and definitively interpreting test results can occur in both lay users and trained professional test users [43]. Misreading a weakly positive strip is obviously restrictive because the subject falsely believes it to be negative. However, the instructions for use clearly state that a negative test does not mean that RDST-Ag is not contagious. The misreading of invalid tests interpreted as positive should, in principle, be corrected by further molecular testing for COVID-19, which should be systematic in case of positivity, as indicated in the instructions for use. Post-test responses to the satisfaction questionnaire on the instructions for use (substudy 1), self-diagnosis (substudy 2) and interpretation of the results (substudy 3),showed that the vast majority of the COVID-19 self-test steps were considered easy or very easy by most participants, as previously reported for HIV self-test using a similar rapid test cassette [28, 30]. It was especially appreciated to see a video before the test. The willingness to repeat the use of the Ag-RDST assay when necessary is important for widespread use, because the Ag-RDT for SARS-CoV-2 detection must be repeated to identify infectious individuals, or for private personal use to be make it a routine. Taken together, our observations highlight the usability of the BIOSYNEX COVID-19 Ag+ Antigen Self-Test in adults using painless NMT self-sampling as a novel approach to assess for SARS-CoV-2 infection using the Ag test. -RDT and self-interpretation. of the results in a supervised environment. The feasibility of community self-collection of nasal swabs has previously been emphasized for the diagnosis of infection with respiratory viruses, such as influenza A and B and other common respiratory viruses, including common coronaviruses [44, 45], and these swabs appear to be both easy to perform [42, 43, 44, 46, 47, 48] and well accepted [46, 48]. Self-collection of nasal secretions for respiratory viruses offers significant potential benefits by reducing the need for personal protective equipment, limiting patient and staff exposure to infection, increasing comfort and access for patients, and timeliness of receipt of the sample [45, 48, 49]. In the present series, the handling of the test itself did not pose any particular problem, as previously demonstrated for other self-tests with similar cassettes [28, 29, 30, 50]. NMT self-sampling was acceptable and achievable at a variety of education levels, and most participants preferred this method over healthcare collection, likely due to patients' ability to control the comfort level of collection. nasal better than a trained. harvester can, as previously reported for other respiratory viruses [46, 47, 48]. Finally, the interpretation of the two bands on the Ag-RDST study strip was generally correct, as previously shown for rapid diagnostic tests to be comparable in lay users of the general adult population [28, 29].
4.2. Lay User Analytical Performance of the BIOSYNEX COVID-19 Ag+ Antigen Autotest
Analytical performance of the Ag-RDST assay was assessed by reference to multiplex rtRT-PCR for the detection of SARS-CoV-RNA. 2 as the gold standard in a real life community setting. In this evaluation, the sensitivity of the BIOSYNEX COVID-19 Ag+ Antigen Autotest was lower among samples from asymptomatic individuals (83.3%) than among samples from symptomatic individuals (93.8%). The specificity (>99.0%) was high in samples from asymptomatic and symptomatic groups. The prevalence of positive SARS-CoV-2 rtRT-PCR test results in this population was relatively high (20.7% overall; 7.9% for asymptomatic participants and 53.3% for symptomatic participants). The high viral load of SARS-CoV-2 in the upper and lower respiratory tracts, including nasal sites [41, 47, 48, 51] and sensitive molecular techniques may explain the equivalent sensitivity of nasal self-collection to samples collected for health care in patients with COVID-19. The Ag-RDST study complied with current WHO recommendations for Ag-RTD screening indicating that, at a minimum, Ag-RDTs should correctly identify more cases than are they would lose (sensitivity ≥80%) and have a very high specificity (≥97-100%) [52]. Furthermore, analytical performances should be of comparable order, as participants in our Ag-RDT study previously reported for some Ag-RDTs in lateral flow immunoassay format [9, 53, 54, 55, 56, 57 , 58, 59, 60, 61], while several studies have reported much lower sensitivity levels that contrast with an always high specificity [6, 62, 63, 64, 65]. In addition, the BIOSYNEX Ag-RDST COVID-19 Ag+ Antigen Autotest complied with the current recommendations of the Haute Autorité de Santé (Saint-Denis, France) for an Ag-RTD screening, indicating that, at a minimum, Ag -RDTs need to correctly identify more cases than would be missed in symptomatic patients (≥80% sensitivity) as well as in asymptomatic individuals (≥50% sensitivity) and must have a very high specificity (≥99%) [66]. Our results clearly show that the analytical performances of the Ag-RDST study were better in the case of high viral load, that is, in the case of significant viral excretion, especially in symptomatic individuals. These observations confirm the differential interest of Ag-RDT for the detection of SARS-CoV-2 antigens depending on the level of viral load of the sample analyzed, underlined by several authors [67, 68, 69] and international [52] and national recommendations [66, 70]. As indicated for Ag-RDT [52, 66, 70], Ag-RDSTs can best be used to detect symptomatic SARS-CoV-2-infected individuals suffering from COVID-19-like symptoms with viral loads therefore they are highly contagious individuals. Previous studies to date have analyzed the performance of supervised and unobserved self-collected samples for the molecular detection of SARS-CoV-2 [45, 71, 72, 73, 74, 75 , 76, 77]. Therefore, Tu and colleagues compared the detection of SARS-CoV-2 using a variety of self-collected swab types (under observation by a healthcare professional) by 530 symptomatic child and adult outpatients (or their parents). or guardians), with nasopharyngeal swabs collected by healthcare personnel. professionals as the gold standard of comparison [72]. Of the nasopharyngeal swabs collected by the healthcare professional, 51 (9.6%) were positive. Self-collected samples from the middle turbinate showed a high sensitivity of 96.2% (87.0% to 100%) [72]. McCulloch et al. They have shown that unsupervised home midnasal swab collection was comparable to physician-collected nasopharyngeal swab collection for molecular detection of SARS-CoV-2 in symptomatic patients, particularly those with higher viral loads [ 71]. High analytical performance of self-collected samples, including NMT samples, for molecular detection of common respiratory viruses has been similarly reported [44, 45, 46, 47, 48, 78]. A meta-analysis of nine studies comparing self-sampling and healthcare worker sampling for influenza testing reported a pooled sensitivity of 87% and specificity of 99% for self-collection [79]. Lastly,The performance of supervised self-collected samples for the detection of SARS-CoV-2 using a WHO-listed SARS-CoV-2 Ag-RDT was evaluated in adult volunteers at the Charité hospital, Universitätsmedizin Berlin, Germany [12]. , 13, 14, 15]. Thus, nasal sampling (including self-sampling) evaluated against nasopharyngeal sampling led to comparable performance with the SARS-CoV-2 Ag-RDT [12, 13, 15]. Klein and colleagues expanded these studies by evaluating the clinical performance of a self-collected, supervised NMT swab and a professionally collected nasopharyngeal swab, using Panbio™ Ag-RDT (distributed by Abbott) in reference to molecular testing [14]. Participants were able to reliably perform NMT self-sampling. A SARS-CoV-2 infection was diagnosed by rtRT-PCR in 45 of 290 participants (15.5%). Comparing NMT and nasopharyngeal sampling, the Ag-RDT percent positive agreement was 88.1% (95% CI: 75.0-94.8%). The sensitivity of Panbio™ Ag-RDT was between 84.4% and 88.9%. The specificity was> 99.0% for NMT and nasopharyngeal sampling. The sensitivity of Panbio™ Ag-RDT in participants with high viral load (>7 log10 SARS-CoV-2 RNA copies/mL) was 96.3% (95% CI: 81.7–99.8 %) for both NMT and nasopharyngeal sampling. Supervised NMT self-sampling returned results comparable to NP sampling. Overall, our findings and Klein's study demonstrate that NMT autosampling leads to results comparable to nasopharyngeal sampling when a suitable Ag-RDT is used.13]. In the same setting, Nikolai and colleagues further demonstrated that professional anterior nasal sampling and NMT had equivalent precision for a SARS-CoV-2 Ag-RDT in outpatient symptomatic adults, but did not evaluate self-anterior nasal sampling for Ag-RDST. [13]. Interestingly, the Centers for Disease Control and Prevention (Atlanta, GA, USA) have recently added both self-sampling for reference laboratory analysis and NMT home self-sampling as an acceptable alternative to sampling. professional nasopharyngeal in their guide to SARS-CoV-2 testing [11]. Direct COVID-19 self-tests and home tests that have received Emergency Use Authorization (EUA) status from the US Food and Drug Administration (FDA) and are on the market, including available at Oral fluid-based self-tests have also been authorized by the FDA under an EUA, although further clinical studies on the sensitivity of saliva as a sample material for COVID-19 self-test are warranted.Other Developing technologies could be used as a self-test. For example, loop-mediated isothermal amplification (LAMP) is a DNA amplification method that allows rapid and sensitive detection of a specific gene. LAMP combined with reverse transcription (RT-LAMP) has been used successfully for the detection of several respiratory RNA viruses, including SARS-CoV-2 [80]. Recently, a low-cost and easy-to-use paper-based device has been developed for extraction-free detection of SARS-CoV-2 in whole saliva using RT-LAMP with a colorimetric response visible to the human eye, which could be used as a self-test or home diagnosis due to its simplicity [81].
Conclusions
Ag-RDST can be used to detect symptomatic SARS-CoV-2-infected individuals suffering from COVID-19-like symptoms with elevated viral loads and has the potential to determine highly contagious individuals [52, 66, 70].
Large-scale detection of populations with active SARS-CoV-2 infection is a possible way to break the chains of transmission to limit the current pandemic and unconfine societies. Although repeated population screening of asymptomatic individuals can be used to limit the spread of SARS-CoV-2, speed of reporting is much more important than sensitivity, because the results related to screening of infected and asymptomatic individuals must always be known. quickly [18].
Therefore, despite lower sensitivity for detecting infections, Ag-RDST for SARS-CoV-2 antigen detection may be an important tool for screening due to its fast turnaround time and lower costs [ 67, 68]. The rapid performance of Ag-RDST can help limit the transmission of SARS-CoV-2 by more quickly identifying infectious people to isolate, especially when used in the context of serial testing strategies. The need for health services has grown around the world during the COVID-19 pandemic. Innovative ways to address this crisis are required. Automatic collection of nasal swabs for SARS-CoV-2 antigen testing offers an acceptable and reliable alternative to samples collected by healthcare workers. Therefore, the collection of swabs associated with Ag-RDST presents several advantages, which are even more important at a time of global health crisis, including accessibility outside the health care system, minimizing the use of personal protective equipment, limiting the exposure of patients and staff to infection and providing a more comfortable experience for the patient. Self-collection for patients infected with SARS-CoV-2 or COVID-19 is well accepted, safe, and scalable in the pandemic setting.16].
The high sensitivities of Ag-RDST, especially in symptomatic patients, alleviate concerns about the increase in false negatives in this setting. As societies reopen, expanded testing becomes critical to prevent a global resurgence of COVID-19.
Nasal self-sampling with Ag-RDST has the potential to play a critical role in increasing access to testing in the general population.
Update of the GTM report on diagnostic tests for COVID-19
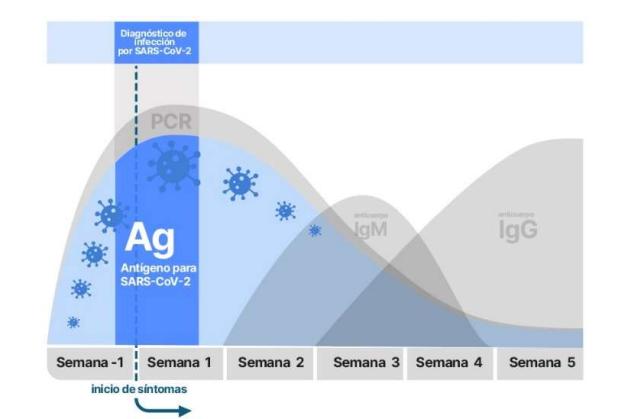
Currently, the diagnostic tests that exist are based on the detection of:1.- Genetic material of the virus2.- Viral antigens3.- Antibodies generated against the virusPCR techniques and those for the detection of genetic material and viral antigens, respectively, are Very well established, they present a high specificity and sensitivity, and allow us to know if these elements of the virus are present at a specific moment, although they do not indicate at what moment of the infection we are. On the other hand, the generation of antibodies by the immune system is a known response that follows a temporal sequence and helps to identify the moment of the infectious process in which the patient is. Also, it allows the identification of individuals who have been hosts of the virus, even when they have been asymptomatic during the infection.
How to Simulate a Hangout #hangout #simulate https://t.co/wkUjLZtMnG
— iAndroid.eu Fri May 29 08:30:33 +0000 2020
1.- DETECTION OF THE VIRUS GENE MATERIAL BY MOLECULAR TECHNIQUES1.1 PCR The reference technique for detecting the presence of the specific gene material of the SARS-CoV-2 virus is the polymerase chain reaction (PCR). The sample used is usually a nasopharyngeal exudate, although saliva, sputum, tracheal aspirate, or bronchoalveolar lavage can also be used. Currently, PCR is the most sensitive and specific diagnostic technique available in this pandemic (https://pubmed.ncbi. nlm.nih.gov/32570045/). PCR specifically detects specific RNA sequences of SARS-CoV-2, both genomic and in the replicative state, from the asymptomatic phase of incubation to several weeks after the resolution of the clinical picture. Despite its high sensitivity, variable false percentages have been reported. Negative due to factors associated with sample collection and processing and viral load dynamics. Regarding the latter, we know that it peaks within the first 7 days of infection, although some infected people may retain genetic material from the virus for weeks and even months, especially in patients who do not resolve the clinical picture. In contrast, positivity detected after resolution of the clinical picture, especially in patients who develop neutralizing IgG antibodies against the virus, is usually associated with minimal or no infectivity. The variable percentage of false negatives is considered to be higher in the first 5 days. after infection (up to 67%), and at least on day 8 (21%), so it would be advisable for PCR tests to be performed 1–3 days after the onset of symptoms in order to minimize false negatives .In addition, in asymptomatic patients, aCRP cycles should be taken into account in which the sample is considered to be infective. Positive PCR at cycles (Ct) greater than 35 after a period of mild symptoms or after the mandatory days of virus incubation quarantine should be considered negligible in terms of clinical significance and patients as non-infectious. The difficulty of interpreting the results in some cases, along with the complexity of obtaining the sample, the laboriousness of the technique itself, and the need to carry it out in specialized laboratories by expert technicians, are some of the limitations of the PCR technique.3 There is an RT-PCR automated, very fast (30 minutes) and working as a Point of Care (POC) (portable instrument), using the Xpert Xpress system (cepheid.com). It comes in a format that does not require specialized facilities or expert technicians. It is designed mainly for emergency situations and urgent diagnostic needs and could also be combined with the detection of other respiratory viruses (to filter false positives). With its use, the saturation of emergency services could be avoided. Thus, the combination of SARS-CoV-2, influenza A and B, and respiratory syncytial virus (Xpert Xpress SARS-CoV-2/Flu/RSV) has been announced in a single test. This equipment is very specific, but it is available in most Microbiology laboratories for urgent detection tests for other viruses and is already being used for SARS-CoV-2.1.2 ISOTHERMAL AMPLIFICATION Isothermal amplification is also based on molecular techniques and, in comparison with PCR requires a constant temperature for the amplification reaction and identification of a fragment of the virus's genetic material. There are numerous variants of this technique (https://pubmed.ncbi.nlm.nih.gov/32093592/). This test is faster than PCR (5 to 30 minutes). This is a very sensitive technique that can therefore have the same interpretation difficulties as those mentioned above for PCR. There are at least five companies that market this technology in the form of Point of Care techniques (portable instrument) that include swabs and extraction kit and do not require expert technicians to perform and interpret them. One of these techniques is the one used by Abbott's ID now commercial model, which is one of the most widespread diagnostic systems based on this technique in the USA. (https://www.globalpointofcare.abbott/es/product-details/id- now.html)Loop-mediatedisothermal amplification or LAMP is a specific isothermal amplification technique almost as sensitive as PCR. On November 17, the FDA approved the first autotest for home use called Lucira based on this technique (https://www.lucirahealth.com/), although it is not yet commercially available and a medical prescription will be required for its use.1.3 CRISPRThis type test, based on the CRISPR system,detects the genetic material of the virus by hybridizing a probe to a specific region of the genetic material of the virus. This, in turn, allows binding of a Cas enzyme to DNA, resulting in activation of a probe that fluoresces upon processing. As it depends on an enzymatic activity, the signal is4 amplifiable and cumulative, which allows a very sensitive detection of the virus's genetic material without the need for its processing. The CRISPR test requires, like isothermal amplifications, minimal manipulation and it is expected that it can become a self-diagnostic test in not too distant dates. Like the previous ones, its high sensitivity can pose diagnostic problems when detecting positives in people who no longer secrete infective virus. Currently, there is only one approved kit that uses this technology. (It is included in a list of approved tests; https://www.360dx.com/coronavirus-testtracker-launched-covid-19-tests) Sherlock Biosciences. Sherlock CRISPR SARS-CoV-2 kit (EUA5/6/2020)EUA: Emergency Use Authorization1.4 Digital PCRTo solve the problem of sensitivity when viral loads are low, several companies are marketing a high-sensitivity PCR technology called digital PCR ( dPCR), based on a highly sophisticated technique that employs microfluidics and droplet-level analysis. Companies such as Gnomegen LLC, PreciGenome LLC, and Bio-Rad market it.1.5 SEQUENCING THE VIRUS Another diagnostic option is direct sequencing of the virus. This technique requires more sophisticated equipment and is mainly used to identify mutations in the SARS-CoV-2 sequence. It is of interest for the study of possible cases of reinfections as well as the evolution of the strains and their possible involvement in the development of the pandemic. Thus, it can detect mutations with clinical repercussions in terms of pathogenicity, evasion against treatments based on antiviral drugs and/or neutralizing antibodies, or the complete neutralizing immunity against the virus that is expected from vaccines. This sequencing technique is what has allowed the rapid identification of the mutations detected in the UK and South Africa, which have greater infectivity, but similar virulence. (See Tables 1A and 1B) 5 ADVANTAGES AND DISADVANTAGES OF MOLECULAR TECHNIQUES Advantages: In general, the RT-PCR test is the mainstay of the diagnosis of COVID-19, due to its sensitivity, specificity and feasibility compared to viral culture. For its interpretation it is necessary to consider the moment of the PCR test in relation to the symptoms, the detection limit of the assay and the type of sample. The next improvements in RT-PCR are aimed at greater ease of processing, saving materials and time delivery of faster results. Allows the use of RNA pools: clinical sensitivity has been shown to be maintained with pools of up to 8 patients. This approach is useful in situations with a lack of reagents and/or a high number of samples, in order to reduce costs and speed up screening processes, especially at times of low incidence.https://www.ncbi.nlm.nih.
- Rapid tests are simple, they do not require sophisticated equipment or highly qualified personnel, but they must be carried out by trained personnel.
- They are faster and cheaper than PCR
- Allows earlier isolation of positive cases.
- May allow rapid massive studies in high-prevalence and/or high-risk situations (for example, residences), and repeat them in people with potential high risk. Disadvantages:
- Most of them have a lower sensitivity than RT-PCR tests
- There are no data in asymptomatic people7
- The positivity window is narrower than the by RT-PCR (Figure 1)
- Negative results in a population with a high prevalence of infection should be confirmed by RT-PCR or repeat the antigen test.
COMPARISON OF RAPID TESTS FOR VIRUS DETECTION Diagnosis of SARS-CoV-2 infection is typically made using standard RTPCR, which requires transportation of the sample to a specialized laboratory as well as specialized personnel, equipment, and materials. Because of this complexity, there is great interest in rapid molecular and antigen tests. Rapid molecular and antigen tests differ from standard RT-PCR in several ways: they facilitate access to testing through point-of-care assays, improve the efficiency as they reduce the process steps between sample collection and results and some also eliminate the need for a central laboratory and specialized equipment. The sample for rapid tests is obtained with a nasal swab, which is generally better tolerated than a nasopharyngeal swab.8 Rapid tests can ultimately contribute to better containment of SARS-CoV-2 through more effective detection and subsequent isolation. Consequently, some hospitals are using or considering using rapid tests as part of their SARS-CoV-2 testing algorithms. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-testsguidelines .html#table2The evaluation of antigen tests for the diagnosis of COVID-19 demonstrate similar specificity to standard RT-PCR tests, but lower sensitivity than rapid molecular tests, which show similar sensitivity to standard RT-PCR tests https ://www.finddx.org/covid19/dx-data/. Rapid antigen and molecular tests can differ in their performance characteristics, making it difficult to extrapolate the evaluation of a specific rapid test to others. Until more reliable data on the use of these tests in clinical settings become available, standard RT-PCR will likely remain the most reliable technique for diagnosing SARSCoV-2 infection. However, in certain scenarios, a rapid test may be a reasonable initial screening test; These include situations where rapid isolation is important, such as in primary care and emergency services or cases where a symptomatic person has had known contact with someone with COVID-19. Thus, no recommendations are made for or against the use of rapid tests (ie, time to result ≤ 1 hour) versus standard RNA tests in symptomatic individuals suspected of having SARS-CoV-2 infection. Several ongoing clinical trials aim to evaluate rapid tests for COVID-19 (https://clinicaltrials.gov/ct2/show/NCT04460690). In the case of the Lucira COVID-19 All-In-One test kit, mentioned above, it is a rapid PCR test that provides results in 30 minutes. 3.- DETECTION OF ANTIBODIES AGAINST THE VIRUS In this case, the antibodies or immunoglobulins generated against alvirus in blood, serum, plasma or saliva samples, mainly. The sensitivity and specificity of these tests depends, above all, on four factors:
Paraskevi Goggolidou
Introduction
Since the onset of the COVID-19 pandemic, case identification through testing has been a key pillar of the global response, with a focus on reverse transcriptase-PCR molecular testing (PCR) for diagnosis. PCR remains the gold standard in all countries, 1 and most countries also use it for diagnostic and surveillance purposes. Although PCR can provide measurement of viral load through cycle threshold, results are still routinely reported as positive or negative, treating all infected individuals in the same manner. 2 Furthermore, while PCR is invaluable in the diagnosis of COVID-19 and could be useful in tracking epidemiological dynamics during an outbreak, 3it has limitations including cost, the laboratory infrastructure that is normally required to perform the test and deliver results in a timely manner.
Rapid antigen detection tests (RADTs), in the form of lateral flow assays, have been recognized as having great potential to address the limitations of PCR, particularly for low- and middle-income countries. 4 They have been developed as laboratory tests that require specialized equipment and as point-of-care tests with easy reading of results and performance. The World Health Organization 5 has published guidance on the use of RADTs and recommends them for administration by healthcare professionals, while the European Center for Disease Control and Prevention ( ECDC) recently published 2 technical reports outlining considerations around the use of self-assessments. with RADT both for asymptomatic people and in occupational settings. 6 , 7Self-assessment is defined as the process by which an individual takes their own nose/throat sample (nasal swab, throat swab, saliva, or a combination of the above) and proceeds to perform the test and interpret the results for yourself. Larremore et al 8, based on their model, have advocated for rethinking current public health strategies in testing by shifting towards scaling up RADTs, with a focus on ensuring repeat use and frequency in all settings and populations in an effort to allow countries to open up their societies and economies.
From a public health perspective, RADTs are extremely useful for identifying active infections. 9 , 10 but also increase the accessibility of individuals to the tests and, thanks to the speed of obtaining the results, allow the early detection of positive cases, also controlling the transmission of diseases. Additionally, the availability of RADT has not only improved convenience for many people who would have had difficulty getting to a testing site, but has also made testing more accessible for those who are vulnerable, protecting themselves, self-isolating, or await elective hospital surgery. 11RADTs, on the other hand, rely on the willingness and ability of the individual to correctly perform the self-test and report a positive result, leading to both underreporting and an increase in the number of false positives and false negatives, according to the epidemiological panorama. 6 Therefore, their misuse could make it difficult to track disease trends over time. Additionally, self-test samples are not available for sequencing and monitoring variants of interest. 6
The reluctance of some countries to try additional detection and diagnostic methods other than PCR has shifted their focus toward large vaccination programs. In a herculean scientific effort, targeted single and double dose vaccines for COVID-19 (Pfizer, Astra Zeneca, Moderna, Johnson & Johnson) have received emergency use authorization from regulatory authorities and are currently being rolled out in everyone. The safety and high levels of efficacy in preventing symptomatic disease demonstrated in randomized clinical trials are being documented from real-life “efficacy” implementation studies in the US, UK and Israel. 12–14 More recent data have also highlighted the role of vaccines, including those from Pfizer-BionTech and Moderna, in preventing asymptomatic infections in vaccinees, 12 although decreased sterilizing immunity has been reported during circulation of the Delta variant. 15 Despite great promise to reduce hospitalizations and mortality, limited quantities of vaccine exist globally, which is hampering our efforts in the race to vaccinate the world. 16 Vaccine hesitancy is also challenging governments in their efforts to get people to accept vaccination at a time of heightened anxiety and pandemic fatigue. 17–20
In Europe, self-assessment has been used as a complementary tool in identifying cases and identifying people who have developed some form of immunity to SARS-CoV-2, allowing people to return to work safely. safe and obtain information on the evolution. of the epidemic, even when a herd immunity threshold has been reached. 21–24 governments in both the UK and Greece have adopted the use of RADT surge tests in an attempt to slowly reopen businesses, schools and retail stores and, more recently, to control outbreaks local variants of SARS-CoV-2, such as delta. -variant (B1.617.2) in UK hotspots. 25 The use of free RADTs for SARS-CoV-2 detection using nasopharyngeal swabs was offered to all businesses in the UK in March 2021, followed by bi-weekly testing for all businesses. asymptomatic people in early April 2021. 24 Similarly, Greece has introduced the use of 2 free nasal RADTs for weekly use by asymptomatic people in schools, businesses and community settings. 26Although the use of lateral flow antigen tests was authorized by the US Food and Drug Administration (FDA) 27 in March 2020, they were initially required a prescription for use by the general public. Two home rapid antigen tests are now sold over-the-counter on pharmacy shelves, without the need for a prescription for asymptomatic people in the US, allowing the country to mitigate the chain of transmission and reduce the prevalence of SARS- CoV-2. 28
As more countries are moving towards different modes of COVID-19 testing by expanding their testing policies, we were interested in investigating the acceptability and feasibility of self-testing. To do this, we conducted a cross-sectional survey of residents in Greece and Cyprus over 18 years of age, with a focus on the island of Lesbos, Greece in the period from January 16 to March 16, 2021 using the JISC online platform, with the in order to determine participant preferences about COVID-19 testing and to identify any relationship between particular demographics and their views on self-assessments.
Methods
Study design and participants
The online survey was created on the JISC platform, which is designed for educational and research institutions (https://www. onlinesurveys.ac.uk/about/ ). The survey was distributed to 1,000 people via email lists and social media using the public URL https://uow-survey.onlinesurveys.ac.uk/covid-19-testing-and-long-term-symptoms and was open in the period 16 January to 16 March 2021. All responses were anonymous and validation of the survey questions and ethical approval was provided by the University of Wolverhampton FSE ethics committee (LSEC/202021/PG / 52).
People aged 18 and over were recruited through the Les Mills Gymnasium Network, Greece and the Paraskevi Goggolidou Academic and Professional Network in Greece and Cyprus. Stratified sampling was used to ensure an adequate gender and age distribution of the online survey. Ten or more responses per age group (18-24, 35-44, 45-54, 55-64, 65+) were required to be included in the study analysis. Emphasis was placed on recruiting participants from people permanently residing in Lesbos, Greece, due to its geographical location and representative environment. Lesbos is the third largest island in Greece, has a manageable population size, and is representative of the Greek demographics. It also has a large refugee integration center and had a steep slope in the number of positive COVID-19 cases at the end of 2020/early 2021.
The survey was open to participants for the duration of the study, but the same email account holder was unable to access it again after completion. The online survey consisted of the participant information sheet and consent, followed by a series of questions comprising: individual demographics and the respondent's ability to employ non-pharmaceutical interventions such as physical distancing during the COVID-19 pandemic ; history of prior COVID-19 testing and disease manifestation; preference in COVID-19 sampling, testing methodology, and settings. A survey map is provided in Supplementary Figure 1. In all cases, where data was missing, this particular individual's responses to all questions were excluded, so only complete responses were considered.
Data analysis
As the outcome measure used categorical data collected, frequencies were used to present descriptive data. Chi-square tests were performed to compare sociodemographic and attitudinal data between willing and unwilling/don't-know groups and for demographic predictors of test preferences. A logistic regression analysis was performed to assess the factors that may predict willingness to self-assess. A p value <0.05 was considered statistically significant.
Results
Of the 1,000 people to whom the survey was distributed, 860 participants accessed the survey online and a response rate of 62% was obtained. Excluding incomplete responses, a total of 248 complete responses were received. About 60% of the respondents were based on the island of Lesbos, Greece, 20% in the rest of Greece and 20% in Cyprus. Accepted responses were balanced by gender distribution (55% female, 45% male) and demographic characteristics unique to the region (97.6% White, 98% Greek as their first language, 72% highly educated, 83.1% Christian , 62% employees; table 1). Approximately 14% of respondents had suspected or confirmed COVID-19 prior to participating in the survey, and 63% had already undergone a professional COVID-19 diagnostic test. Approximately 62% reported that they had not been able to maintain social distancing, 59% worked in confined spaces, and 26% were a close contact of a Covid-19 case (data not shown).
Table 1. Summary of the demographics of the participants. View larger version
Most participants (79%; n = 196) reported being willing to self-assess and the remaining individuals reported not (10.5%; n = 26) or don't know (10.5%; n = 26 ). For data analysis purposes, the don't know and don't know groups were combined (21%; n = 52) into one group (don't know/don't know group). A Pearson's chi-square test was performed to measure whether there were significant demographic differences between the want and don't know groups. The analysis revealed that those who are willing to self-assess are more likely to be college graduates than those who were in the don't know/don't know groups (χ 2 = 15.398, df = 1, p <0.001 ). No other demographic differences were found between the wanted and don't/don't know groups (Table 2, results for significant variables only).
Table 2. A summary of the significant differences between the groups that were willing to perform a COVID-19 self-test at home and those that responded No / Don't know. View larger version
Logistic regression models were performed on significant chi-square variables to measure predictors of willingness to self-assess. Being a college graduate significantly predicted the likelihood of being willing to self-assess (odds ratio [OR] = 3.455, P 2 = 8.95, df = 3, P <0.03); graduates were more likely to prefer the saliva test and less likely to prefer the fingerstick test than nongraduates (Table 3). Furthermore, chi-square found that non-Greeks were significantly more likely to prefer the saliva test and less likely to prefer the nasal swab than Greeks (χ 2 = 9.12, df = 3, P 2 = 3.652, df = 3, P > .05).
Table 3. Pearson's chi-square test found significant differences between college graduates versus non-graduates and Greeks versus non-Greeks in preferred type of COVID-19 test.View Larger Version
Notably, the majority of participants would prefer to test once a week (40%; n = 100), <1 in 5 of those surveyed chose to test once a month (19 %; 46), while around 1 in 3 responded that they never wanted to be tested (31%; n = 76). Pearson's Chi-square test found significant differences between the 2 groups; those who were willing to self-assess preferred once a week, while those who reported no/don't know preferred never (Table 2). Finally, more than half of the participants would prefer to test at home (52%; n = 129) or had no preference at the test site (22%; n = 54). Pearson's chi-square test found significant differences between the 2 groups (χ 2 = 36.331, df = 6, P <0.001); the willing group preferred to be tested at home, while the don't/don't know group preferred home, private clinic, or EODY/YDY settings (Table 2).
Discussion
Our data show a general preference for self-testing for COVID-19 in the least invasive way, with a preference for saliva as the biological material of choice. In current self-assessment practices, nasal swabs are generally purchased. 29 Saliva should be considered as an additional biological sample for COVID-19 testing, as although its sensitivity is lower than that of PCR with a swab, 30 is a clinically acceptable material 31and its ease of use allows simple and repeatable testing, increasing the probability of detection of positive cases. In addition, the non-invasive nature of saliva acquisition allows sample collection in children, people who are disabled, vulnerable or anxious, and where resources are scarce and, while having limitations, may increase the uptake of repeat testing. 29
The concept of self-assessment was very new at the time we designed our survey. Although our study was cross-sectional in nature, the survey population was limited, and an overrepresentation of college graduates was observed in the survey population, our findings give us insight into individuals' preference for self-assessment. The survey was conducted in an adult population that represents the demographics of the country, the only barrier to participation being access to a technological device. This was addressed with the help of local volunteers who helped 7 participants without online access to complete the questionnaires. Some participants also initially submitted incomplete responses; where this was evident, our local volunteers helped them resubmit a full response.32 and as such, these findings only reflect the primarily Greek population. In the future, this limitation could be addressed by diversifying the points from which participants accessed the survey, as this could have improved the diversity of the sample population. Other limitations of the study include the fact that it was completed before the asymptomatic self-screening test was launched in Greece; It is possible that after repeated exposure and twice weekly nasal testing, participants' preferences about testing frequency and method may have changed. Our study did not access the preferences of people under the age of 18, so no conclusion can be drawn about their views on self-assessment.
The use of self-assessment RADTs could play a vital part in helping a government cautiously lift restrictions to open up its economy and society. 6, 7, 30 It can also be critical during a rapid increase in cases, as demonstrated most recently in India, where government guidance indicates that a reactive antigen home test is a definite positive. 33 As of March 31, 2021, the US Food and Drug Administration (FDA) had authorized 4 tests for use over-the-counter without a prescription for asymptomatic serial testing for SARS- CoV-2. 34 In Greece, self-assessment RADTs have been distributed to all registered citizens through pharmacies since the beginning of April 35 and in the UK an approach was carried out similar for the self test twice a week. 24 The use of an online system for reporting reactive COVID-19 results in a lateral flow assay is essential and has been successfully implemented by the NHS through the Trace and Contact app in the UK. 36 People with a reactive result for COVID-19 in Greece report it through an online platform. 37 In addition to testing, contact tracing should complement case identification, as it makes it possible to break transmission chains by quickly identifying clusters or outbreaks in specific settings, 38 sup> and even if positive cases do not formally report or misreport their result, they may inform their contacts, which can have a positive impact on transmission control.
Thus, while self-testing can improve a country's response to COVID-19 even as vaccination coverage increases, a number of factors need to be considered before self-testing can be scaled up universally and reliably. . In Europe, manufacturers must demonstrate compliance with the applicable legal requirements of the EU Directive 98/79/EC for in vitro diagnostic medical devices, in order to be able to place a RADT on the market. 39 Concerns have already been raised about the high false negative rate in RADT with sensitivities of 72% and 58% observed in cohorts of symptomatic and asymptomatic participants, respectively. In addition, a recently published Cochrane review reported variations in sensitivities between brands ranging from 34% to 88% 40 and another study investigated RADTs with promising performance characteristics, identifying Innova RADT as one of those. tests with excellent specificity. 11 As the market for over-the-counter test kits expands rapidly through government-supported self-testing programs, the ability to centrally register and assess the efficacy of different antigen tests it becomes paramount.
For mass self-testing approaches to be successful in breaking chains of transmission, countries will need to focus on end users, provide testing widely and freely, and build trust in the process. Our findings show that 52% of participants prefer to get tested at home compared to alternative settings and 31% are reluctant to get tested due to financial cost. Easily accessible and freely available self-assessment would be a strategy to address these barriers. In order to achieve meaningful results, users will need to receive clear instructions on how to perform the self-assessments and some key members of the community will need to be trained and act as ambassadors for the self-assessments. In addition, it will be necessary to take a proactive approach when reporting a result, either through the adoption of purpose-built applications or through a dedicated and easily accessible website. In Greece, since the free self-testing was launched, 42 million RADTs were distributed to 4.6 million citizens and about 85,000 positive cases were confirmed by PCR.41 Different countries have different practices on the validation of self-test results and our recommendation is that a reactive result should be validated by PCR, in a way that does not harm or cause harm to the individual or society; Self-sampling and home collection of samples for validation could be a solution. It should also be noted that while the number of false positives will vary depending on the baseline prevalence, 8 , 9 the success of this approach will depend on the population participation that can be stimulated by the campaigns. of education and community ambassadors, the effective and timely notification and validation and the provision of state support to positive cases during the period of quarantine and isolation.
Conclusions
Although vaccine-induced immunity is the vehicle to control and end the pandemic, self-testing for COVID-19 as part of a comprehensive testing approach should be prioritized in parallel as Vaccination campaigns are advancing in all countries. Adequate monitoring and evaluation of RADT implementation should be done through self-tests, where test metrics are shared quickly and publicly, as has been done with country vaccination rollouts. Importantly, the RADT self-test should be understood as a screening strategy, rather than a diagnostic test, while saliva should be considered as additional sampling material, especially in cases of young, vulnerable, and hard-to-reach populations. A screening approach using repeated use of RADTs, even by self-assessment, has clear benefits over what is considered lower analytical sensitivity of the assay compared to more expensive molecular diagnostic tests. Pandemics require innovation and boldness in policy development. Expanding testing to include self-testing may be critical to keeping the incidence of COVID-19 at lower levels, especially as we move into the next phase of the global COVID-19 response in an era of increasing vaccination coverage, although limited.
Share it:
I like this:
Like Loading...Related

- 783
- an atlanta preferred women's health center
Related Articles
How many sit-ups should I do a day to get rid of my belly?
30/01/2022Flattening the abdomen or reducing the fat in this area is a difficult task. But don't despair, we will solve the question: how many sit-ups should I do a day to eliminate the belly. Abdominal fat...
Jalisco The Ministry of Health invites men and women to learn about family planning services
04/02/2022The Jalisco Ministry of Health invites men and women of reproductive age to learn about and use the various services offered by the Family Planning and Contraception Program, which...
Telva International day against gender violence: screens multiply mistreatment against women
01/02/2022SaludUpdated Change of scenery, same victims. On the International Day for the Elimination of Violence against Women, we highlight how aggressions have been copied to the digital sphere, increasing...
Coronavirus Spain today | | Health The Trust Project
18/03/2022The Ministry of Health has reported 3,261 new coronavirus infections and 155 deaths, while the cumulative incidence drops more than six points in 24 hours to 109.3 cases. There is...